
Your new Crohn’s disease drug improves disease control, but is efficacy enough?
It’s time to get steroid-sparing in the label
The development pipeline is swelling with new drugs and therapeutic strategies aimed at reducing steroid doses or eliminating the need for steroids altogether. These include innovative biologics and highly targeted therapies that selectively modulate the immune system.
The ability to quantify change in glucocorticoid-toxicity plays a central role in cost-efficient development of safe, effective and commercially viable new drugs, therapeutic strategies and steroid-sparing alternatives to the standard of care.
The Glucocorticoid Toxicity Index (GTI) is the first validated clinical outcome assessment (COA) that measures glucocorticoid toxicity directly. The GTI and its siblings (pGTI and GTI-MD) provide a powerful advantage for cost-effective product design and evidence-gathering at multiple points in the development cycle, including:
- Target Product Profile (TPP) to specify a product that will be safe, effective and commercially viable
- Patient stratification for clinical trial recruitment efforts
- Critical endpoint in clinical trials to demonstrate drug candidate safety, efficacy and performance relative to alternative treatments
- Risk prediction modeling and treatment monitoring to identify at-risk patients who may benefit from earlier initiation of glucocorticoid-sparing therapies
Aim to win: include glucocorticoid-toxicity scores in your target product profile (TPP)
Incorporating glucocorticoid-toxicity scores into your target product profile (TPP) – as early as possible in the development cycle – is essential to drive critical design decisions, establish appropriate clinical trial parameters, and generate the evidence you need to demonstrate safety, efficacy and performance relative to conventional therapies and competitor products.
In addition to helping regulators assess efficacy and safety, glucocorticoid-toxicity scores specified in the TPP ultimately determine whether “steroid toxicity reduction” can be included as a claim on product label.
Advance your research with the STOX Suite
The STOX® Suite enables accurate measurement of steroid-toxicity. The STOX Suite includes four clinical outcome assessments (COA) optimized for different settings:
- The GTI: calculates change scores across nine health domains in adults
- The GTI-MD: quantifies change across four metabolic domains in claims and EMR data
- The pGTI: captures the change scores in ten health domains in children
The STOX Suite is the first collection of validated clinical outcome assessments (COAs) of steroid-toxicity. Each COA can be licensed alone or as a bundle for both retrospective and prospective applications. These applications include:
- Clinical trials
- Academic studies
- Health economics and outcomes research (HEOR)
- Real-world experiences
- Clinical practice
Stay on target: the GTI as a key endpoint in clinical trials
Including the Steritas GTI, GTI-MD or pGTI as an endpoint in prospective clinical trials will demonstrate whether your drug candidate is on track to reduce, prevent or even reverse toxicity. The ADVOCATE trial, a randomized, double-blind, placebo-controlled trial of avacopan for ANCA-associated vasculitis, capitalized on the GTI to great effect.
ADVOCATE was the first Phase 3 clinical trial to use the GTI to measure the ability of an investigational agent to reduce steroid-toxicity compared to standard of care.[1] The trial demonstrated that avacopan was non-inferior to a standard prednisone taper for remission induction at 26 weeks. One of the important ways that avacopan differentiated itself from standard treatment, however, was through its reduction in steroid-toxicity as early as 13 weeks, the first time the GTI was measured during the trial. This advantage was maintained at 26 weeks, and reduction in steroid-toxicity was ultimately reported as the most important secondary outcome measure for efficacy.
The CWS and AIS reveal lower steroid-toxicity for avacopan vs. prednisone
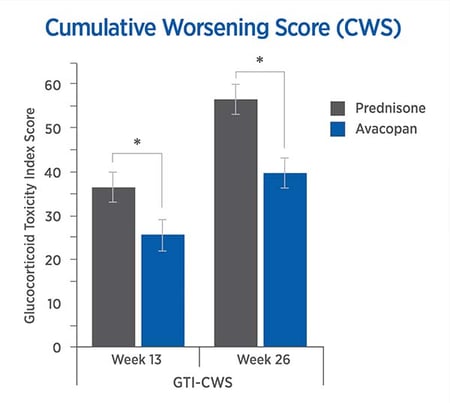
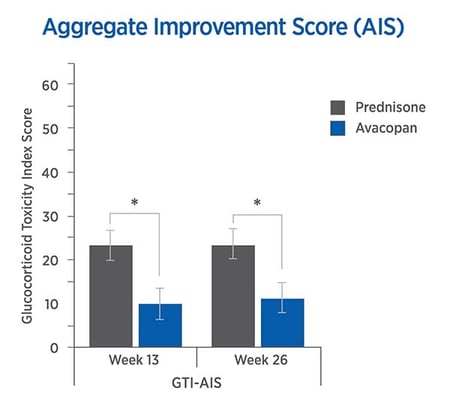
For each trial participant, data were entered into the GTI to generate the Cumulative Worsening Score (CWS), and the Aggregate Improvement Score (AIS), at weeks 13 and 26. The lower scores in the avacopan group confirmed lower steroid-toxicity.
The mean CWS and AIS were both lower in the avacopan group.

"The damage caused by steroid exposure is not surprising - it is expected."
Deborah Gelinas, MD
Neuromuscular Expert Medical Affairs for Argenx

"Trial sponsors have a great opportunity and challenge to demonstrate what they mean by steroid-sparing and how that translates into healthcare benefits such as reduced hospitalizations, and improved quality of life."
Sudhakar Sridharan, MD
Vice President in Medical Science & Strategy Division at PPD/ThermoFisher

"It's very clear that being able to measure things is essential if we want to change them. With the GTI, the pGTI and the GTI-MD, we can measure steroid-toxicity in both clinical trials and clinical practice."
John H. Stone, MD MPH
Professor of Medicine at Harvard Medical School, and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital

"Nobody disputes the value of glucocorticoids, and while most clinicians recognize the damage they cause to patients, they don’t recognize the impact they have on clinical research.”
Andreas Reiff, MD
Chief Medical Officer of Neutrolis

"The GTI is the first and only quantitative measure of steroid-toxicity - It allows us to quantify the scale of adverse reactions, helps us taper steroids, and look after our patients more effectively."
Wen Zhang, MD PhD
Professor Rheumatology at Peking Union Medical College Hospital (PUMCH), Beijing, China

"The GTI is being used in many trials around the world and the development of the GTI-MD should enable the majority of rheumatologists to quantify glucocorticoid toxicity in day-to-day practice."
Frank Buttgereit, MD
Professor of Rheumatology and Deputy Head of the Department of Rheumatology and Clinical Immunology at the Charite University Medicine (CCM) in Berlin
Surge in adoption of the STOX Suite of Clinical Outcomes Assessments (COA)
In response to substantial investment in research and digitization of its COAs, Steritas has seen a surge in licensing and deployment of the STOX Suite. This reflects the growing recognition of the importance of measuring and monitoring steroid-toxicity in pharmaceutical research and clinical practice respectively.
The Steritas STOX Suite (GTI, GTI-MD, and pGTI) has been used in over 75+ clinical trials, in 1100 sites across 80 countries, underlining the growing impact that quantitative steroid-toxicity assessment is having on clinical development in inflammatory diseases.
Steritas is keen to support studies that align with its goal of reducing the impact of steroid treatment on patients; 90% of license applications to date have been successful.

References
- Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. Avacopan for the Treatment of ANCA-Associated Vasculitis. N Engl J Med. 2021 Feb 18;384(7):599-609. DOI: 10.1056/NEJMoa2023386


