
The first validated clinical outcome assessment of the metabolic domains of steroid-toxicity
Steritas GTI-MD
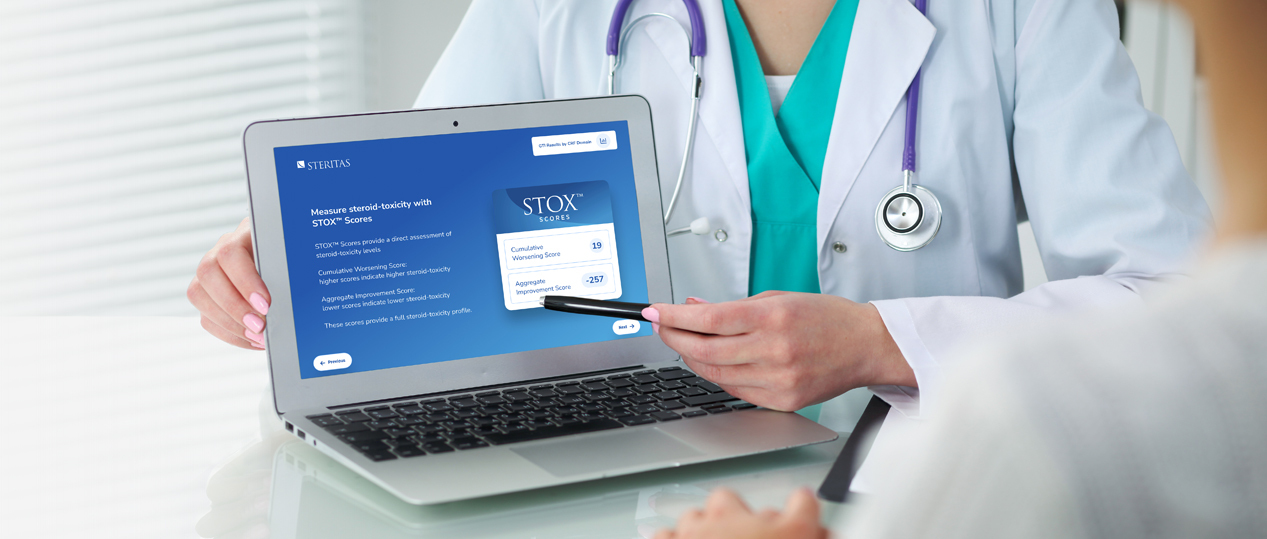
Validated clinical outcome assessment of steroid-toxicity
The Steritas GTI-MD is an abridged clinical outcome assessment (COA) that provides a systematic approach to assessing steroid-toxicity in the clinic, and existing population datasets using data points that are captured in routine clinical practice.
The GTI-MD is fast, accurate and easy to integrate at the point-of-care, via electronic medical record systems, or health economics outcome research programs.
Studies have shown the GTI-MD can reliably identify clinically relevant steroid-toxicity across multiple disease cohorts, and confirm the GTI-MD correlates well with the parent GTI.[1]
Using the GTI-MD in digital health applications can also allow patients to track their own GTI-MD scores, motivating them to actively participate in their treatment by advocating for the lowest therapeutically effective dose of steroids.[1]
Steroid-toxicity in clinical practice
In clinical practice, inducing and maintaining remission of inflammatory diseases often requires long-term steroid treatments that increase the risks of steroid-related adverse events.[5,6] While efforts are made to minimize the impact of steroid-toxicity, clinicians are faced with the unenviable task of balancing disease remission against long term toxicities.
To complicate matters further, clinicians grapple with high patient volume, limited insurance coverage, the need to manage patients with complex diseases, and multiple comorbidities that limit time for additional evaluations.[1]
The GTI-MD works in the background in EMR systems using data already collected by the office staff. The clinician is only alerted when the GTI-MD algorithm detects emerging steroid-toxicity, thus limiting the burden of using it in the clinical setting.
Measuring steroid-toxicity in population research
The GTI-MD is of particular utility for health economics outcome research and population studies - enabling researchers to analyze large claims and electronic health record datasets to understand how glucocorticoid use and cumulative dose are associated with increased steroid-toxicity. This provides a foundation for assessing the benefits of non-steroid alternative treatments.
Through quantitative analysis of treatment costs associated with the 80+ known steroid-toxicities, researchers can use GTI-MD for retrospective data analysis to develop a more accurate picture of how glucocorticoids affect patients outside of controlled clinical trials and anecdotal data collected at the point of care.
"Steroid toxicity is rampant and it can be fatal - bringing it onto the radar screen of the practicing physician is really, really important."
James T. Rosenbaum, MD
Chief of Ophthalmology emeritus at the Legacy Devers Eye Institute, Portland, Oregon

"There has to be an acceptance that steroids are now an undesirable part of treating most rheumatic diseases, and our prescribing behavior must change."
Michelle Petri, MD MPH
Professor of Medicine at the Johns Hopkins University School of Medicine

"Every milligram that helps control the disease is a good milligram, but every milligram we can spare is an even better one."
Frank Buttgereit, MD
Professor of Rheumatology and Deputy Head of the Department of Rheumatology and Clinical Immunology at the Charite University Medicine (CCM) in Berlin
Designed for ease-of-use, whatever your workflow
The GTI-MD balances scientific rigor and data analytics with ease of use. In most cases, the STOX® Suite calculates steroid-toxicity using data that are collected routinely in clinical trial or in practice.
References
- Patel NJ, Jayne, DRW, Merkel PA, Bekker P, Zhang Y, McDowel PJl, Johal J, Heaney LG, Murrel D, Stone MN, Yue H, Stone JH, The Glucocorticoid Toxicity Index-Metabolic Domains, an abridged version of the Glucocorticoid Toxicity Index: post-hoc analysis of data from the ADVOCATE trial, The Lancet Rheumatology, Volume 5, Issue 7, 2023, e413-e421, DOI: 10.1016/S2665-9913(23)00131-5.

