Measure steroid-toxicity using the STOX Suite
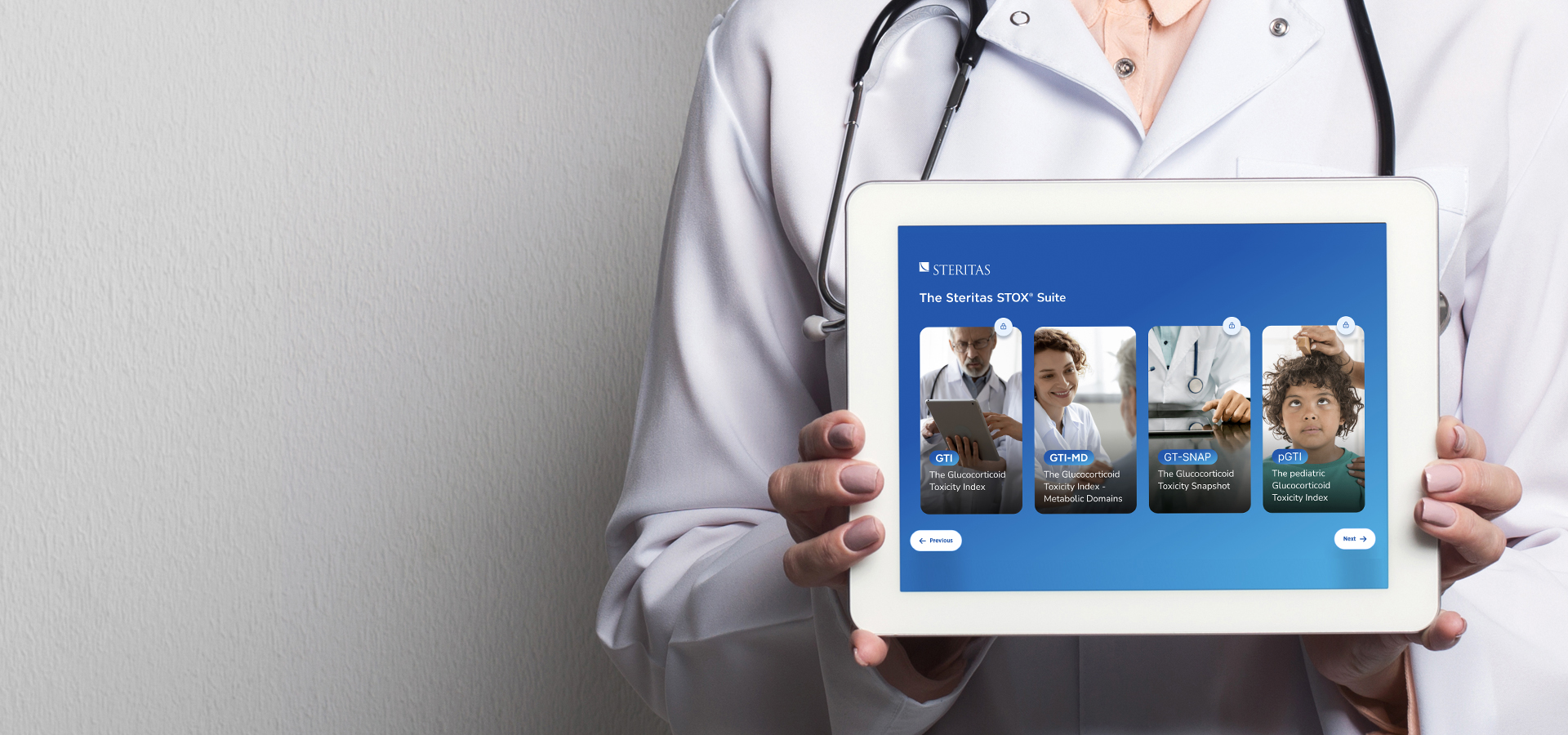
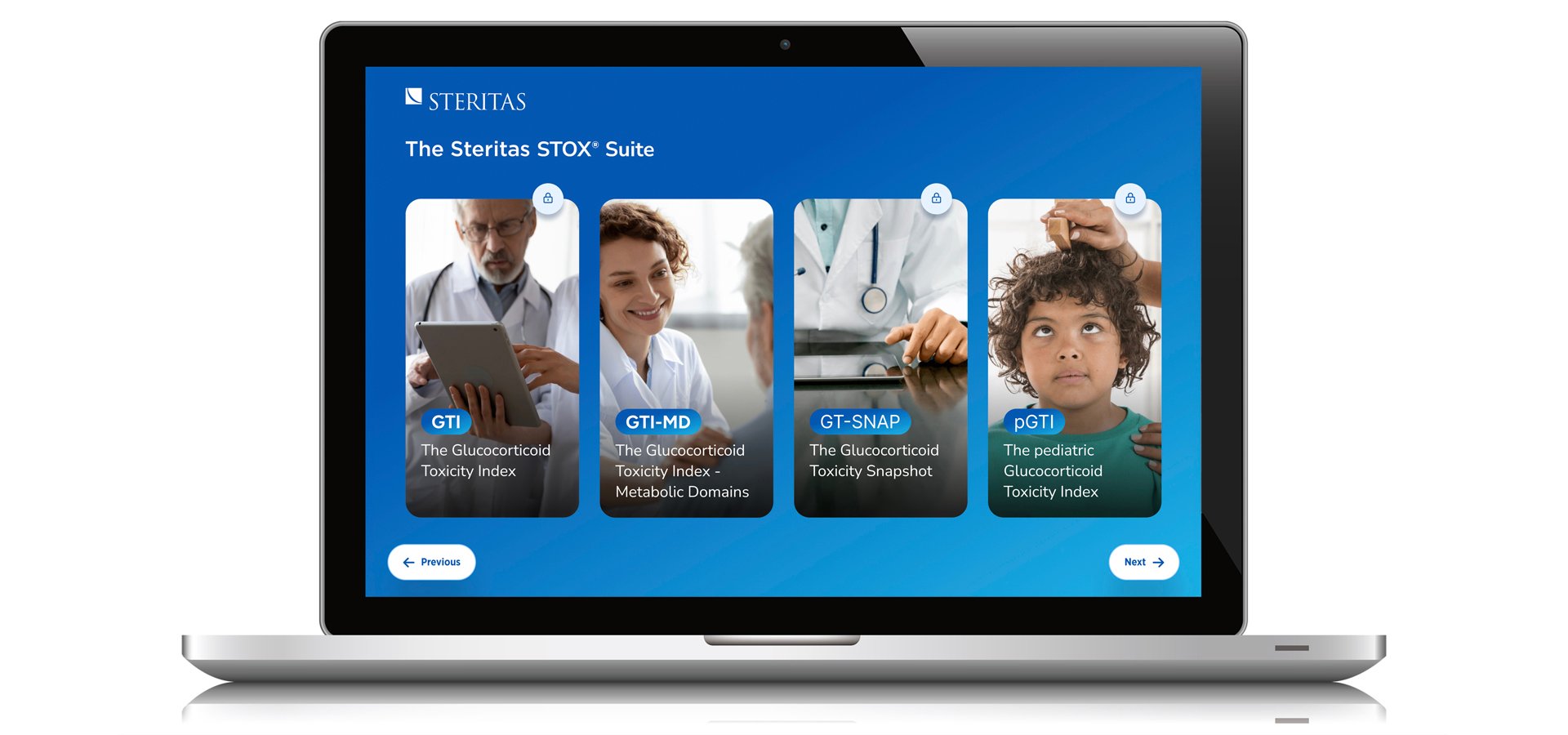
The STOX Suite
The first validated clinical outcome assessments of steroid-toxicity in children and adults
At Steritas we believe that the way to shift steroid prescribing patterns and drive the development of steroid-sparing alternatives is to enable direct measurement of steroid-toxicity in research and at the point of care — for the well-being of patients.
The STOX® Suite of clinical outcome assessments (COAs) was developed to make this possible.
First-in-class digital instruments validated to quantify steroid-toxicity
Since the introduction of the Steritas Glucocorticoid Toxicity Index (GTI) in 2017, there has been a marked surge in the number of clinicians and clinical researchers licensing our growing suite of steroid-toxicity COAs.
The STOX Suite includes:
These instruments provide weighted outcome scores of steroid-toxicity that enable clinicians and regulators to evaluate the impact of steroid-sparing treatments. They are fast, accurate and easy to implement.
Get steroid-toxicity reduction in the label
The instruments that form the STOX Suite have found use in:
- Clinical Trials: to demonstrate the steroid-sparing benefits of new drugs and treatment protocols.
- Population Studies: to deliver actionable insights found in healthcare and payer datasets.
- Point of Care Assessments: to enable early detection of steroid-toxicity that prevents lasting damage to patients and reduces chronic cost to health systems.
- Patient Engagement: to equip patients with ways to engage in steroid-related healthcare decisions that affect their wellbeing.
The GTI, its pediatric sibling the pGTI, and the point-of-care friendly GTI-MD provide a systematic approach to assessing steroid-toxicity, and have now been used in more than 25 disease indications, across more than 1100 sites in 80 countries.

"A version of the GTI that could be built into the EPR and generate a big red pop-up that takes up the whole screen and urges providers to record data and to think differently about the prescription would provide the wake-up call that is so often needed."
Michelle Petri, MD MPH
Professor of Medicine at the Johns Hopkins University School of Medicine

"The damage caused by steroid exposure is not surprising - it is expected."
Deborah Gelinas, MD
Neuromuscular Expert Medical Affairs for Argenx

"Trial sponsors have a great opportunity and challenge to demonstrate what they mean by steroid-sparing and how that translates into healthcare benefits such as reduced hospitalizations, and improved quality of life."
Sudhakar Sridharan, MD
Vice President in Medical Science & Strategy Division at PPD/ThermoFisher

"Having a standardized and validated measure of steroid-toxicity is so important а it enables you to track individual patients' progression and gives validation to the scope of the problem that patients, clinicians and healthcare systems face."
Lisa Christopher-Stine, MD MPH
Professor of Medicine and Neurology at Johns Hopkins University School of Medicine. Director and Co-Founder of the Johns Hopkins Myositis Center

"In recent years, we have seen more and more steroid-sparing therapies being developed - the GTI will help make more steroid-sparing therapies available to us, and give us even more options to reduce steroid-toxicity."
Wen Zhang, MD PhD
Professor Rheumatology at Peking Union Medical College Hospital (PUMCH), Beijing, China
STOX Suite licenses are tailored to the design of your clinical trial.
We welcome your interest in the STOX Suite of steroid-toxicity outcome assessment instruments. All uses of these instruments require a license, whether you are an academic researcher, biotech start-up, a large pharmaceutical company, or a clinical research organization.
As part of each use license, statisticians and data managers access certified scores via the STOX digital platform, an FDA 21 CFR Part 11 enabled software stack classified as a medical device (SaMD).
Steritas will structure a use license to support the scope and design of your trial and the needs of your organization.
Steroid-toxicity measurement in clinical trials
Steroid-toxicity is being measured in a growing list of clinical trials for inflammatory disease drug candidates.
In response to substantial investment in research and digitization of its COAs, Steritas has seen a surge in licensing and deployment of the STOX Suite. This reflects the growing recognition of the importance of measuring and monitoring steroid-toxicity in pharmaceutical research and clinical practice respectively.
The STOX Suite has now been used in over 80 clinical trials and in 1100 sites, underlining the growing impact that quantitative steroid-toxicity assessment is having on clinical development in inflammatory diseases.





