
Steroid-toxicity
is predictable; quantifying it is finally possible
Equip yourself for the regulatory journey with the STOX Suite: validated solutions for steroid-toxicity assessment
Modern medicine is increasingly setting its sights on steroid-sparing alternatives to minimize or avoid the adverse effects of long-term glucocorticoid therapy.
Rigorous safety and efficacy testing is crucial to demonstrate that next-generation treatment protocols and highly-targeted therapies are superior to glucocorticoids.
The use of a validated metric to quantify steroid-toxicity is of increasing importance as new therapies and drug candidates move through clinical development.
The STOX® Suite is becoming an essential tool to help ease the regulatory journey by assessing steroid-toxicity to:
- Demonstrate safety and efficacy of new inflammatory disease treatments
- Aid timely progression through major development milestones
- Support labeling claims
- Conduct post-approval monitoring
Add the STOX Suite to your regulatory strategy
At Steritas, we’re paving the way for more efficient development and commercialization of safe and effective steroid-sparing therapies.
he STOX Suite, our growing range of clinical outcome assessment instruments, includes:
- The GTI: calculates change scores across nine health domains in adults
- The GTI-MD: quantifies change across four metabolic domains in claims and EMR data
- The pGTI: captures the change scores in ten health domains in children
The STOX Suite is the first collection of validated clinical outcome assessments (COAs) of steroid-toxicity. Each COA can be licensed alone or as a bundle for both retrospective and prospective applications. These applications include:
- Clinical trials
- Academic studies
- Health economics and outcomes research (HEOR)
- Real-world experiences
- Clinical practice
The STOX Suite has been licensed in 30+ disease indications, across 2500 sites in 92 countries across the world.
ADVOCATE trial investigators reported the GTI as the most important secondary endpoint of efficacy
The GTI played a central role in the pivotal Phase 3 clinical trial (the ADVOCATE trial) to demonstrate that avacopan (Tavneos®) reduces glucocorticoid exposure and lowers glucocorticoid-induced side effects.
This was the first demonstration in a worldwide randomized, double blind placebo controlled trial to show a clear clinical benefit of such a highly targeted therapy for the treatment of autoimmune and inflammatory disease.
Based on this evidence, the European Alliance of Associations for Rheumatology (EULAR) updated its recommendations in 2022 to propose avacopan as a steroid-sparing alternative for the management of ANCA-associated vasculitis (AAV).[1]
The GTI reveals lower steroid-toxicity for avacopan vs. prednisone
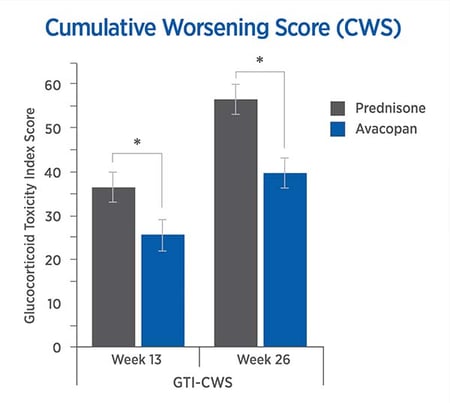
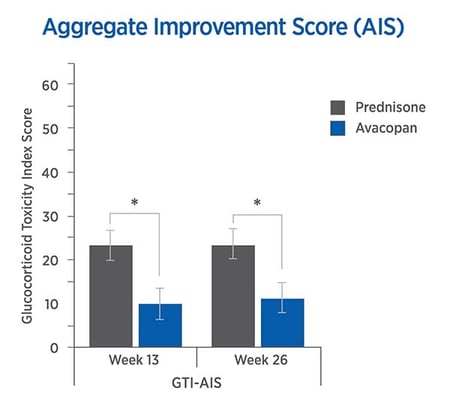
For each trial participant, patient data were entered into the GTI to generate the Cumulative Worsening Score (CWS), and the Aggregate Improvement Score (AIS) for each participant at study weeks 13 and 26. A lower score corresponds to lower toxicity for both indices.
The mean CWS and AIS for all trial participants are both statistically significantly lower for avacopan than for prednisone.

"There has to be an acceptance that steroids are now an undesirable part of treating most rheumatic diseases, and our prescribing behavior must change."
Michelle Petri, MD MPH
Professor of Medicine at the Johns Hopkins University School of Medicine

"The impact of steroid-toxicity is huge - for example in ANCA-associated vasculitis, after five years, steroid-related damage rivals the damage that would have been caused by the disease itself."
David Jayne, MD
Clinical Nephrologist at Addenbrooke's Hospital, Cambridge UK

"It's very clear that being able to measure things is essential if we want to change them. With the GTI, the pGTI and the GTI-MD, we can measure steroid-toxicity in both clinical trials and clinical practice."
John H. Stone, MD MPH
Professor of Medicine at Harvard Medical School, and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital

"Being able to reduce glucocorticoids is a huge benefit to patients as many of the toxicities can cause permanent damage such as joint destruction, diabetes or severe infections that can be fatal in at-risk patients."
Paul Brunetta, MD
Head of Clinical & Translational Science, Sana Biotechnology
Adjunct Associate Professor, Pulmonary and Critical Care Division,
University of California, San Francisco

"The regulatory agencies have been prescriptive in asking for sponsors to present data demonstrating the reduction in steroid-related side effects in order for them to grant a steroid-sparing claim."
Sudhakar Sridharan, MD
Vice President in Medical Science & Strategy Division at PPD/ThermoFisher
References
- Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update Annals of the Rheumatic Diseases, Published Online First: 16 March 2023. doi: 10.1136/ard-2022-223764


