Measuring toxicity of prednisone vs. avacopan for the treatment of ANCA-associated vasculitis
Glucocorticoid-tapering through the trial
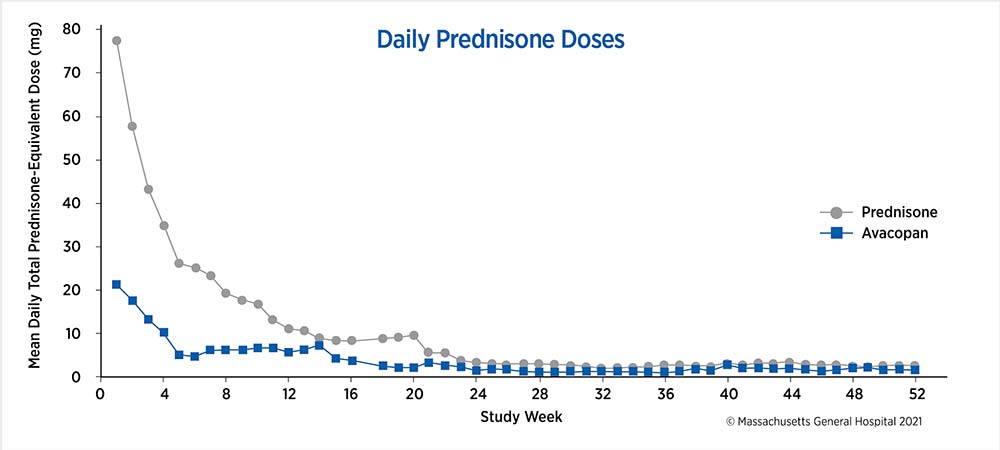 The weekly mean values were calculated based on all recorded systemic (oral or intravenous) glucocorticoid use by all patients in the respective treatment group at the start of each study week.
The weekly mean values were calculated based on all recorded systemic (oral or intravenous) glucocorticoid use by all patients in the respective treatment group at the start of each study week.
Avacopan treatment yields reduced toxicity in all but one of the steroid-toxicity domains used in the GTI
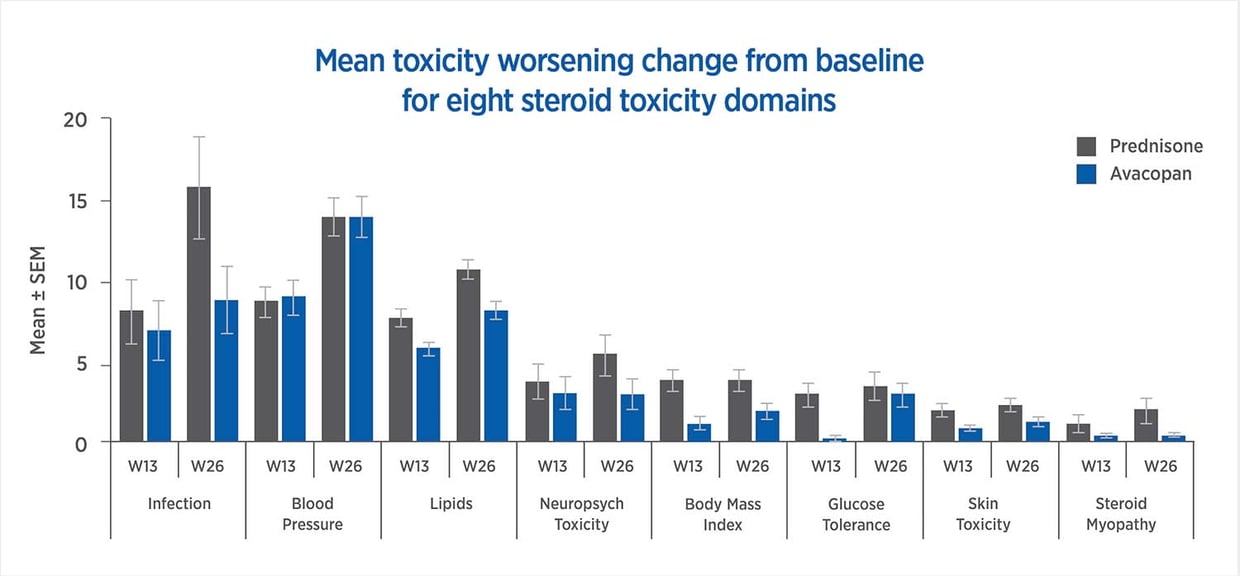
The GTI, CWS, and AIS reveal lower steroid-toxicity for avacopan vs. prednisone
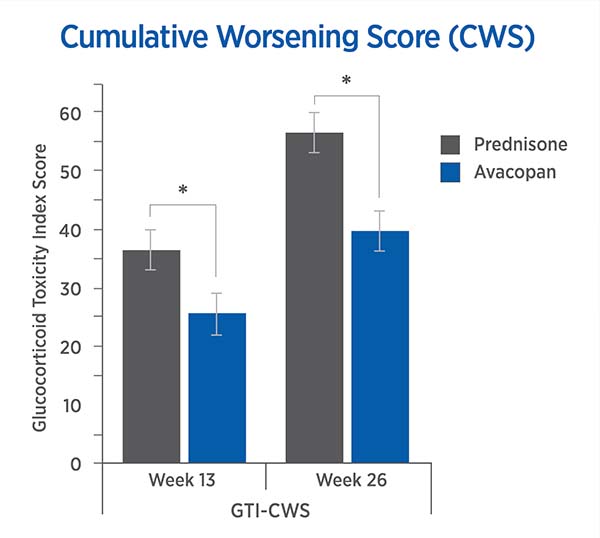
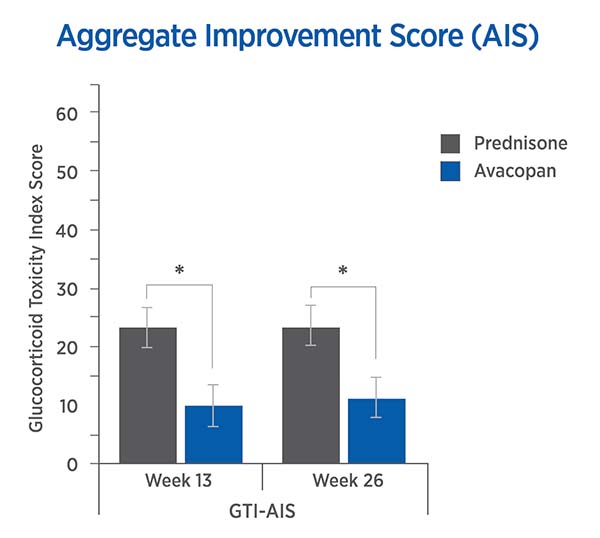
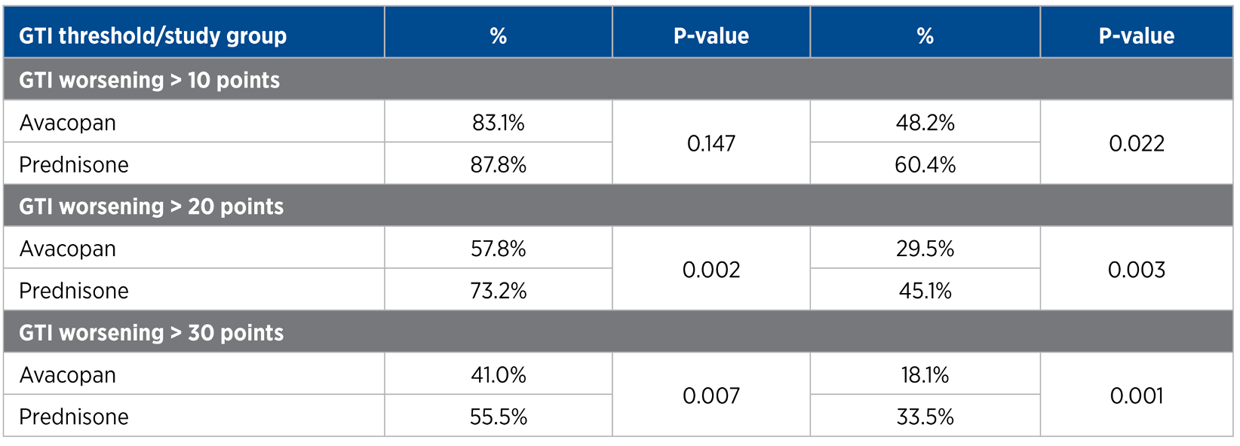
For each trial participant, patient data was entered into the GTI to generate the Cumulative Worsening Score (CWS), and the Aggregate Improvement Score (AIS), at study weeks 13 and 26. A lower score corresponds to lower toxicity for both indices.
The mean CWS and AIS for all trial participants are both statistically significantly lower for avacopan than for prednisone.
Primary and Key Secondary End Points
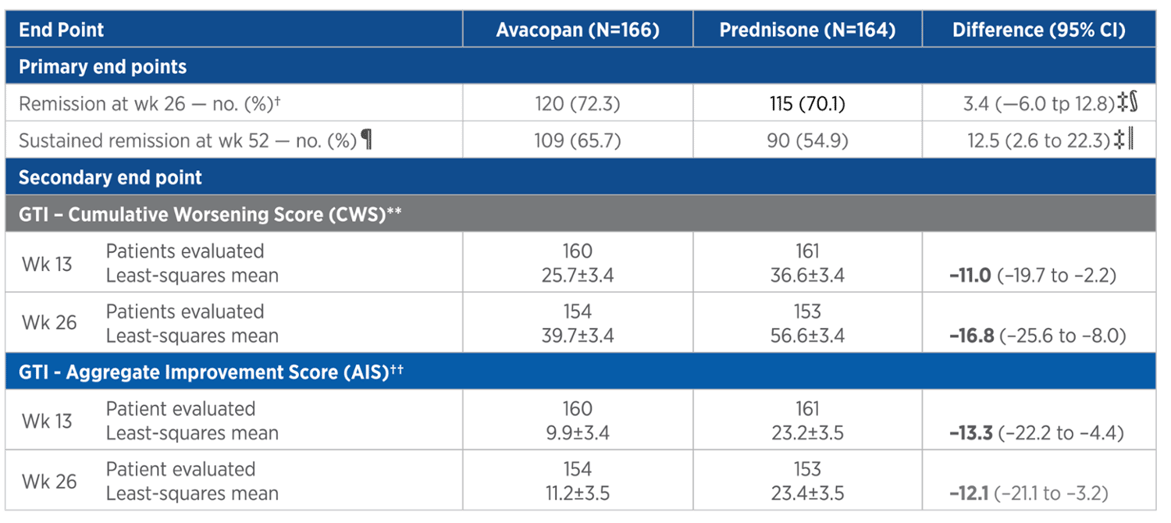
GTI threshold/study group
Percentages of patients in ADVOCATE trial exceeding selected Steritas GTI thresholds at week 26
CWS and AIS reveal statistically and clinically significant differences between treatment groups



GTI point differences between avacopan added, and prednisone only treatement groups:
Cumulative Worsening Score (CWS)
13 weeks: -11.0 points (P = 0.01)
26 weeks: -16.8 points (P = 0.0002)
Aggregate Improvement Score (AIS)
13 weeks: -13.3 points (P = 0.003)
26 weeks: -12.1 points (P = 0.008)
As described by a paper published in The Journal of Allergy and Clinical Immunology in early 2021, the Minimum Clinical Important Difference MCID is 10 points* In the ADVOCATE study, the GTI-CWS difference between avacopan-added and prednisone-only treatment groups at 13 and 26 weeks was -11 and -16.8 points respectively. The GTI-AIS difference between treatment groups at 13 and 26 weeks was -13.3 and -12.1 points respectively. According to the MCID, these values are both statistically significant and clinically important. With this data, the ADVOCATE investigators were able to use the GTI results to conclude that, at 13 and 26 weeks, avacopan reduced glucocorticoid toxicity compared to standard-of-care.

