.png)
Let's face it,
.png)
Let's face it,
now that steroid-toxicity can be measured...
.png)
Let's face it,
now that steroid-toxicity can be measured...
prescribing patterns need to change
.png)
Let's face it,
now that steroid-toxicity can be measured...
prescribing patterns need to change
50 million people1
1% of the world's adult population is taking long-term glucocorticoids.
Top 10 most prescribed2
Oral steroids are in the top 10 most prescribed medications in the United States.
40% more cost3
Steroid-toxicity is linked to 40% of cost of healthcare for patients on glucocorticoids.
Glucocorticoids are overused and toxic: The Great Taper is on
For over 75 years, the world has been hooked on steroids as the first-line treatment for a wide range of conditions caused by inflammation. More than 50 million people (1% of the world’s adult population) take steroids long-term, and it is now clear that the burdens of steroid-toxicity outweigh many of their beneficial effects over time.
The medical community agrees: now is the time to taper steroids and seek steroid-sparing alternatives. At Steritas, an IQVIA business, our mission is to alter steroid prescribing patterns through a public health initiative we call The Great Taper®. The suite of tools we have developed to measure steroid-toxicity is a game-changer in this global effort.
STOX Suite adopted globally to counter the hidden epidemic of steroid-toxicity
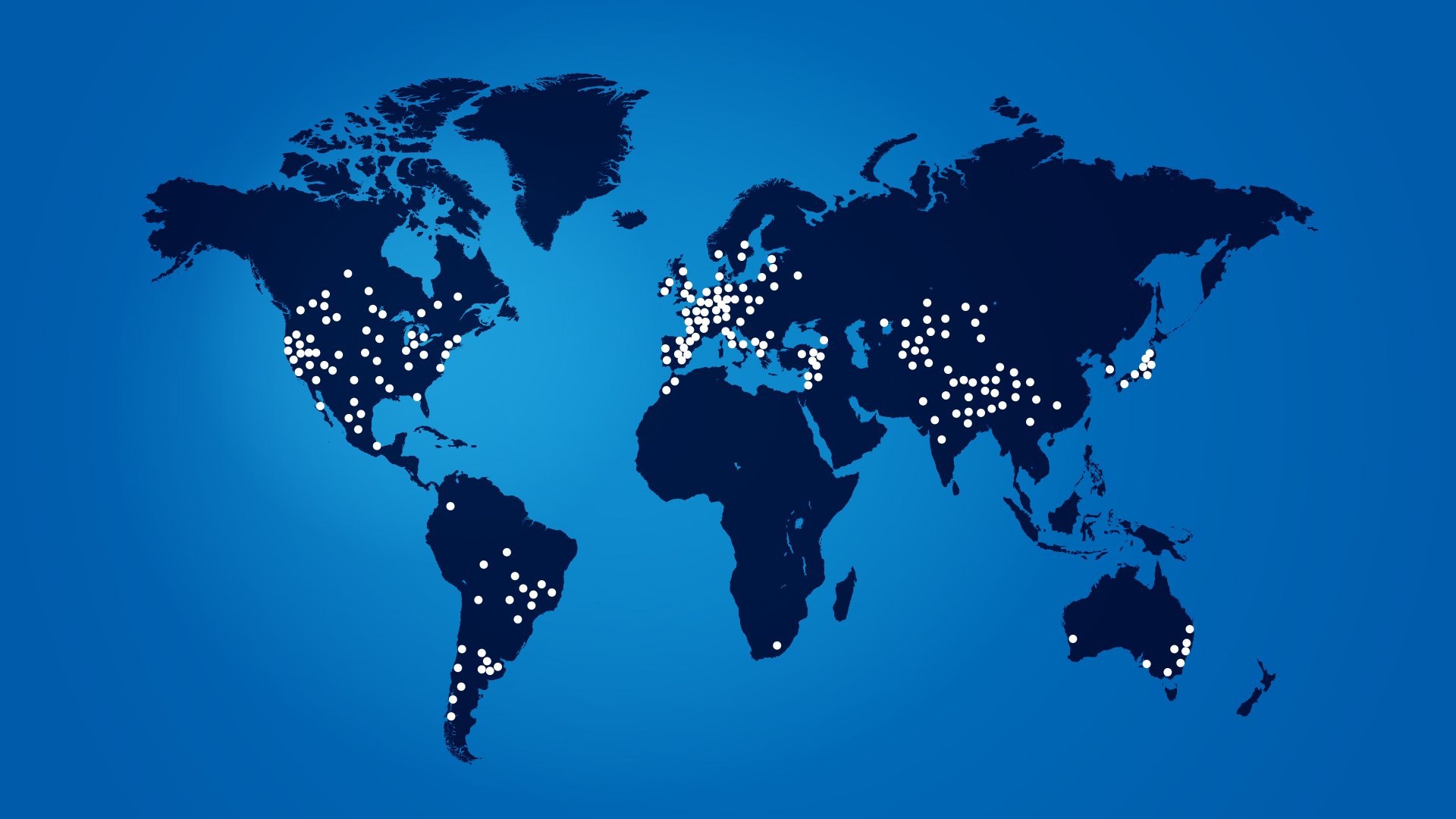
The STOX Suite
The first validated Clinical Outcome Assessments to measure steroid-toxicity
Our growing suite of tools is designed for use in:
- Clinical research for the development of new steroid-sparing drugs and treatment protocols.
- Health and economics outcomes research (HEOR) for the discovery of steroid-toxicity and hidden costs found in large datasets.
- Routine practice to monitor emerging steroid-toxicity in clinic patients.
- Direct-to-patient applications that help patients collaborate in their treatment.
The STOX® Suite provides a systematic approach to assess steroid-toxicity, comprising:
- The GTI: calculates change scores across nine health domains in adults
- The GTI-MD: quantifies change across four metabolic domains in claims and EMR data
- The pGTI: captures the change scores in ten health domains in children
These instruments have now been used in more than 25 disease indications across more than 1100 sites in 80 countries.

"Trial sponsors have a great opportunity and challenge to demonstrate what they mean by steroid-sparing and how that translates into healthcare benefits such as reduced hospitalizations, and improved quality of life."
Sudhakar Sridharan, MD
Vice President in Medical Science & Strategy Division at PPD/ThermoFisher

"The GTI is the first and only quantitative measure of steroid-toxicity - It allows us to quantify the scale of adverse reactions, helps us taper steroids, and look after our patients more effectively."
Wen Zhang, MD PhD
Professor Rheumatology at Peking Union Medical College Hospital (PUMCH), Beijing, China

"Steroids have a role in treating so many diseases - and yet, they do not cure any of them."
John H. Stone, MD MPH
Professor of Medicine at Harvard Medical School, and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital

"The GTI is being used in many trials around the world and the development of the GTI-MD should enable the majority of rheumatologists to quantify glucocorticoid toxicity in day-to-day practice."
Frank Buttgereit, MD
Professor of Rheumatology and Deputy Head of the Department of Rheumatology and Clinical Immunology at the Charite University Medicine (CCM) in Berlin

"Anywhere that steroids are being used in clinical studies, especially randomized controlled studies, there is a place for the GTI."
Paul Brunetta, MD
Chief Medical Officer Hinge Bio
References
- Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatol Oxf Engl. 2011;50:1982–1990.
- https://clincalc.com/DrugStats/Drugs/Prednisone
- Laurence Fardet, MD, PhD, Irene Petersen, PhD, and Irwin Nazareth, MD, PhD Monitoring of Patients on Long-Term Glucocorticoid Therapy- A Population-Based Cohort Study. Medicine. Volume 94, Number 15, April 2011.










